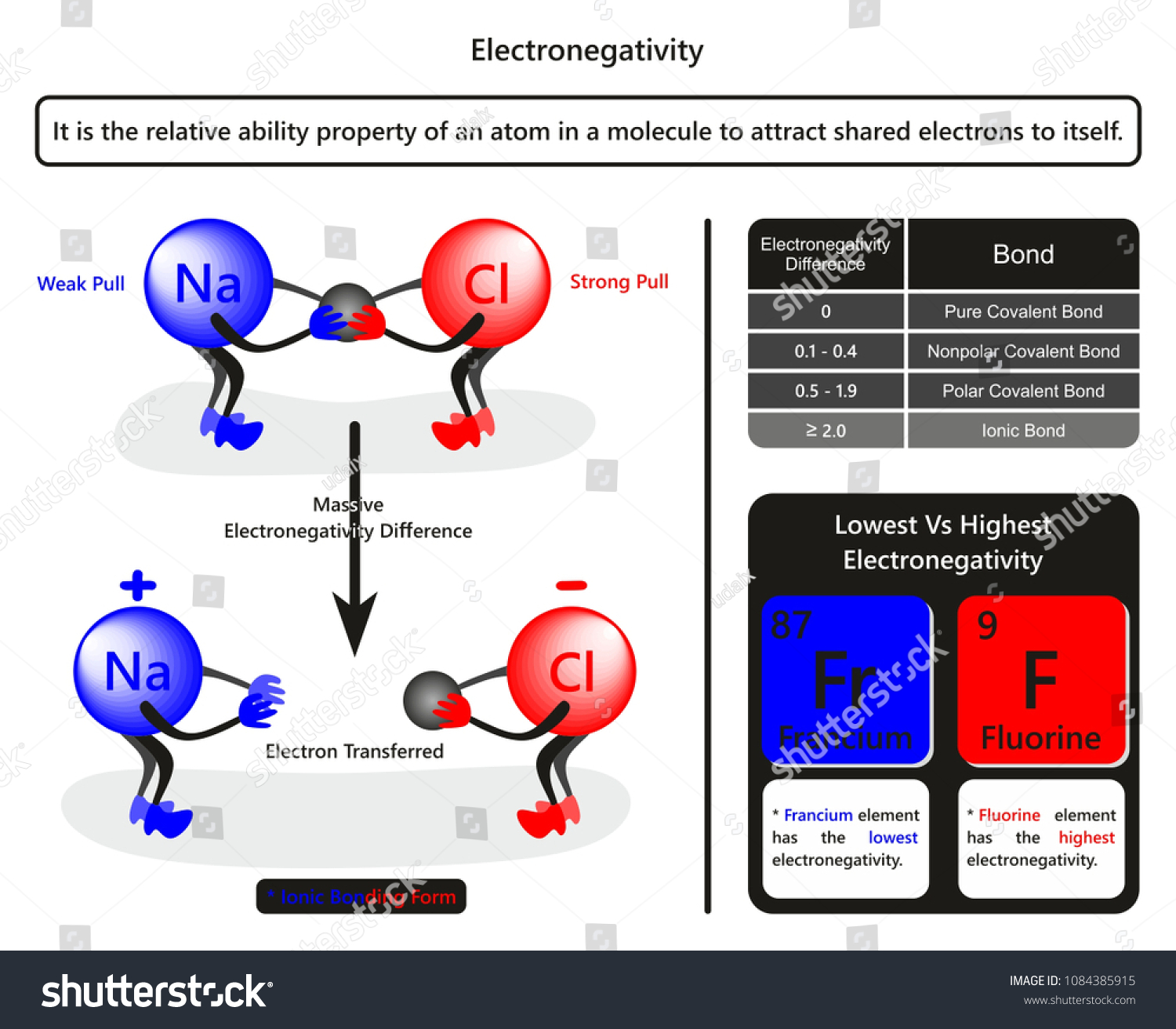
Electronegativity Chlorine Higher Than Sulfur . what is electronegativity? It can also be used to predict if the. use the links in the electronegativity column of the table below for definitions, literature sources, and visual. What happens if two atoms of equal electronegativity bond together? electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. How is electronegativity related to bonding. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. The suggested values are all. How to find electronegativity values. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent.
from avopix.com
3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It can also be used to predict if the. The suggested values are all. How to find electronegativity values. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. use the links in the electronegativity column of the table below for definitions, literature sources, and visual. What happens if two atoms of equal electronegativity bond together? what is electronegativity?
Electronegativity infographic diagram with Royalty Free Stock Vector
Electronegativity Chlorine Higher Than Sulfur It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. use the links in the electronegativity column of the table below for definitions, literature sources, and visual. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. How to find electronegativity values. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The suggested values are all. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 3.16 is the electronegativity value of chlorine (cl). It can also be used to predict if the. What happens if two atoms of equal electronegativity bond together? what is electronegativity? elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. How is electronegativity related to bonding.
From www.slideserve.com
PPT Section 6.5 “Polar Bonds and Intermolecular Forces” PowerPoint Electronegativity Chlorine Higher Than Sulfur How is electronegativity related to bonding. It can also be used to predict if the. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. elements with a high electronegativity (χ ≥ 2.2 in figure. Electronegativity Chlorine Higher Than Sulfur.
From sciencenotes.org
Electronegativity Definition and Trend Electronegativity Chlorine Higher Than Sulfur The suggested values are all. It can also be used to predict if the. 3.16 is the electronegativity value of chlorine (cl). How to find electronegativity values. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. What happens if two atoms of equal electronegativity bond together? what. Electronegativity Chlorine Higher Than Sulfur.
From studydiscretion.z13.web.core.windows.net
How To Determine Electronegativity Difference Electronegativity Chlorine Higher Than Sulfur What happens if two atoms of equal electronegativity bond together? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? elements with a high electronegativity (χ ≥. Electronegativity Chlorine Higher Than Sulfur.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Electronegativity Chlorine Higher Than Sulfur use the links in the electronegativity column of the table below for definitions, literature sources, and visual. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is a chemical property that. Electronegativity Chlorine Higher Than Sulfur.
From learnwithdrscott.com
Electronegativity Table Easy Hard Science Electronegativity Chlorine Higher Than Sulfur It can also be used to predict if the. What happens if two atoms of equal electronegativity bond together? How is electronegativity related to bonding. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a chemical property that measures. Electronegativity Chlorine Higher Than Sulfur.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Electronegativity Chlorine Higher Than Sulfur It can also be used to predict if the. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 119 rows electronegativity is. Electronegativity Chlorine Higher Than Sulfur.
From mungfali.com
Electronegativity Trend Periodic Table Electronegativity Chlorine Higher Than Sulfur 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. How to find electronegativity values. The suggested values are all. It belongs to the 7th group and 2nd period on the periodic. Electronegativity Chlorine Higher Than Sulfur.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Electronegativity Chlorine Higher Than Sulfur elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. How is electronegativity related to bonding. It can also be used to predict if the. It belongs to the 7th group and. Electronegativity Chlorine Higher Than Sulfur.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Electronegativity Chlorine Higher Than Sulfur use the links in the electronegativity column of the table below for definitions, literature sources, and visual. elements with a high electronegativity (χ ≥ 2.2 in figure 2.12.2) have very negative affinities and large ionization potentials,. How is electronegativity related to bonding. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic. Electronegativity Chlorine Higher Than Sulfur.
From www.semanticscholar.org
Figure 2 from Electronic Properties and Dissociative Photoionization of Electronegativity Chlorine Higher Than Sulfur electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 3.16 is the electronegativity value of chlorine (cl). How is electronegativity related to bonding. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It can. Electronegativity Chlorine Higher Than Sulfur.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Electronegativity Chlorine Higher Than Sulfur 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. How to find electronegativity values. electronegativity is a chemical property that measures how likely an atom. Electronegativity Chlorine Higher Than Sulfur.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Electronegativity Chlorine Higher Than Sulfur use the links in the electronegativity column of the table below for definitions, literature sources, and visual. It can also be used to predict if the. How is electronegativity related to bonding. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 3.16 is the electronegativity value of chlorine (cl). How to. Electronegativity Chlorine Higher Than Sulfur.
From www.numerade.com
SOLVED For the compounds HCI and SO2, with the element information Electronegativity Chlorine Higher Than Sulfur 103 rows electronegativity is not a uniquely defined property and may depend on the definition. use the links in the electronegativity column of the table below for definitions, literature sources, and visual. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether. Electronegativity Chlorine Higher Than Sulfur.
From www.slideshare.net
Electronegativity part two Electronegativity Chlorine Higher Than Sulfur What happens if two atoms of equal electronegativity bond together? use the links in the electronegativity column of the table below for definitions, literature sources, and visual. How is electronegativity related to bonding. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if. Electronegativity Chlorine Higher Than Sulfur.
From www.nuclear-power.com
Electronegativity Pauling Scale Electronegativity Chlorine Higher Than Sulfur It can also be used to predict if the. How to find electronegativity values. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. what is electronegativity? 3.16 is the electronegativity value of chlorine (cl). use the links in the electronegativity column of the table below for definitions, literature. Electronegativity Chlorine Higher Than Sulfur.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Electronegativity Chlorine Higher Than Sulfur use the links in the electronegativity column of the table below for definitions, literature sources, and visual. 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or. Electronegativity Chlorine Higher Than Sulfur.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Chlorine Higher Than Sulfur The suggested values are all. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? How is electronegativity related to bonding. use the. Electronegativity Chlorine Higher Than Sulfur.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Electronegativity Chlorine Higher Than Sulfur 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if the. use the links in the electronegativity column of the table below for definitions, literature sources, and visual. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a chemical property that measures. Electronegativity Chlorine Higher Than Sulfur.